
Handling
An introduction to effective handling techniques for labratory rodents.
The biggest external factors influencing the outcome of an in vivo scientific study is the handler. The experience and confidence of the handler is one aspect of this but also important is the experience of the animal and the extent to which the handling by a human generates a stress response. Handling is a necessary part of the management of laboratory rodents to ensure their wellbeing and for health monitoring but also as part of the scientific procedures. In this section we focus on the handling of animals for procedures, but this is closely aligned with habituation methods in the next section.
Table of Contents
- Mouse Handling
- Rat Handling
- Methods which can be used to facilitate restraint in mice but which avoid the use of tail handling
- Methods which can be used in rats to facilitate substance administration via the intraperitoneal or subcutaneous route of administration, but which avoid physical restraint
- Dosing in palatable solutions
- Training for oral dosing using palatable solutions
Impacts of restraint on animal welfare and cumulative suffering – physical restraint is widely used in rats and mice and considered a necessary part of some husbandry procedures and many of the scientific procedures the animal will experience. Standard methods of restraint in mice usually involve using a ‘scruff’ technique where the animal is restrained by the base of the tail and then the skin behind the neck and along the back pinched into a scruff to physically restrain the animal. A similar method is also sometimes used in rats although many now prefer a two-person method where one performs the restraint and the other undertakes the procedure. Physical restraint is known to cause distress (for both animal and handler) but many consider there is no alternative either for handler/animal safety and/or the nature of the procedure. Most rats and mice display overt signs of distress and repeated exposure to physical restraint is an established model of depression. Reducing or avoiding physical restraint has the potential to achieve major welfare benefits through:
- Reduced cumulative suffering – each physical restraint causes distress so reducing the number of times an animal is restrained will impact on their lifetime experience.
- Changing the association between the human handler and the animal – where physical restraint can be avoided or paired with a positive experience, there is the potential for animals to form positive (see habituation) rather than negative associations with human handling. This benefits the science by reducing potential confounds associated with the stress response and reduces variability in the data.
- More translational animal models – stress has a huge impact on all functions in the body and contributes to mortality, morbidity and will influence most scientific outputs. Chronic stress causes a host of adaptive changes which are known to affect the brain, the immune system, development etc. This in turn impacts on the value of the animal model and particularly the relevance to human disease.
- Improved handler experience – researchers and technical staff find these methods reduce their stress and anxiety when carrying out procedures.
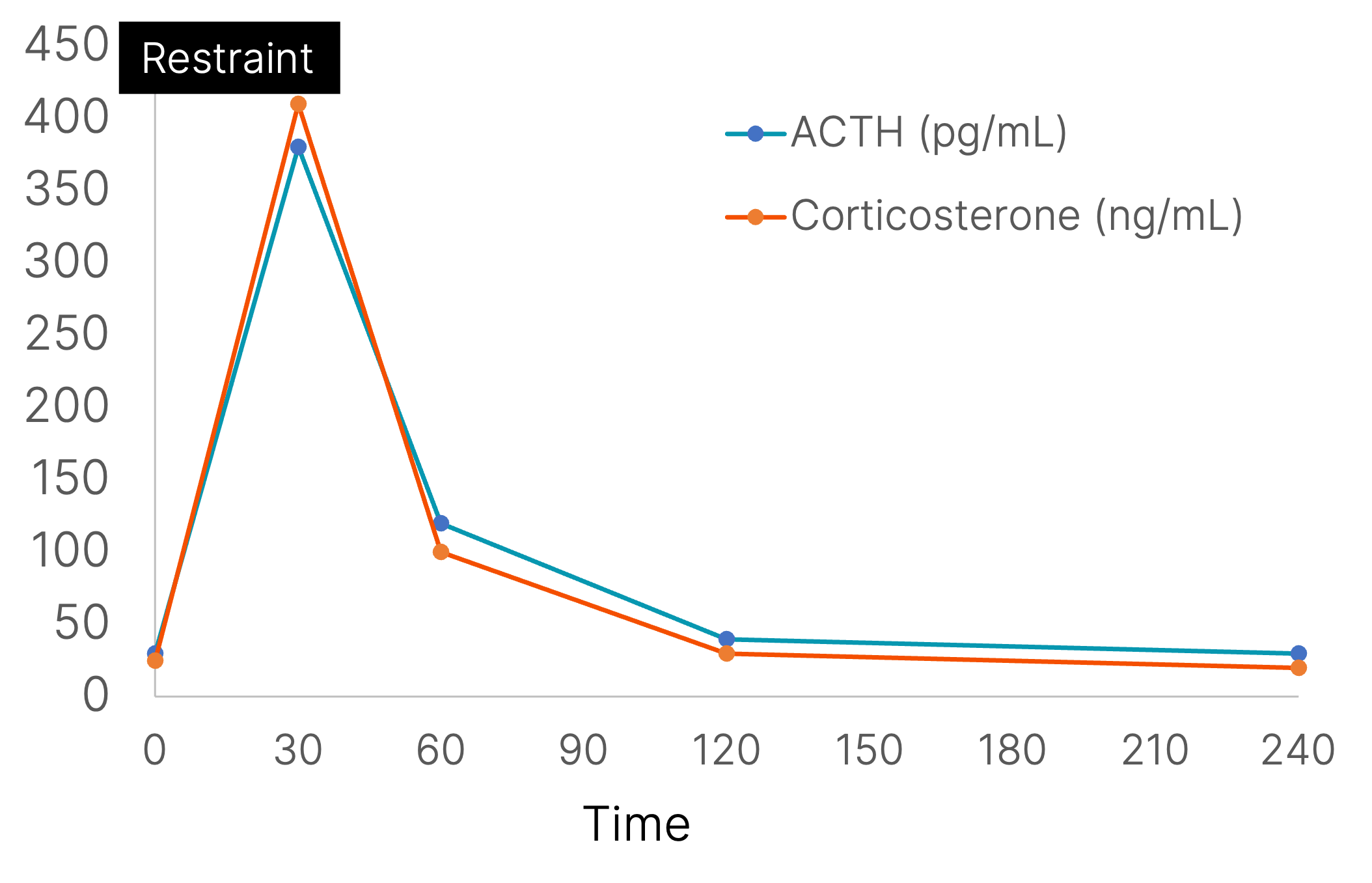
Acute restraint increases the stress hormone, corticosterone. In this re-drawn figure, the effects of restraint on plasma corticosterone and adrenocorticotropic hormone (ACTH) levels are illustrated. The period of restraint is illustrated by the solid box at the top of the graph. (data taken from McClennen et al., (1998) Endocrinology 139(11):4435-41.)
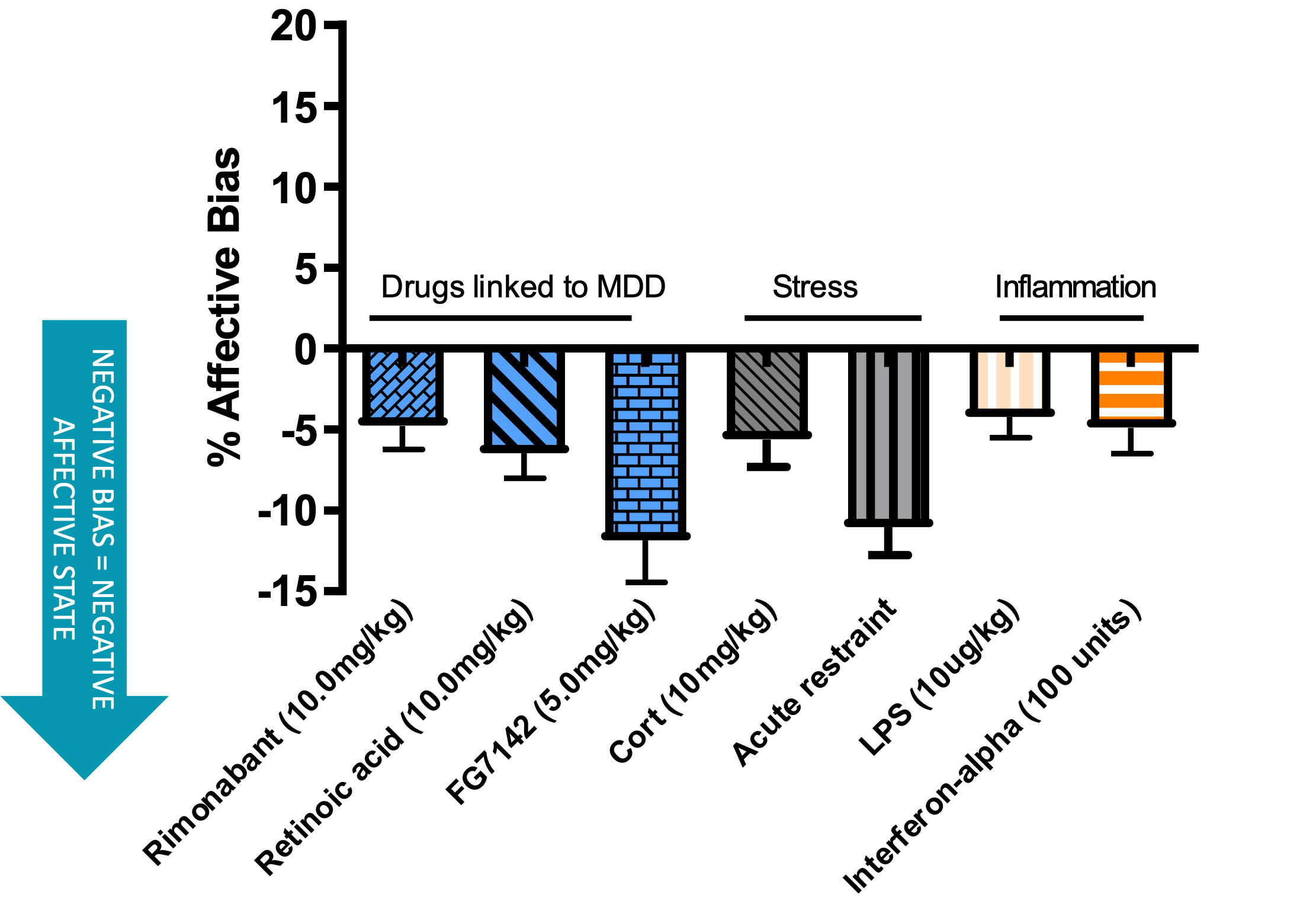
Acute restraint induces a negative affective state of a similar magnitude to an anxiogenic drug. These data were obtained using the affective bias test (see additional resources).
Highlighted in red is the data obtained following a 10 minute restraint stress.
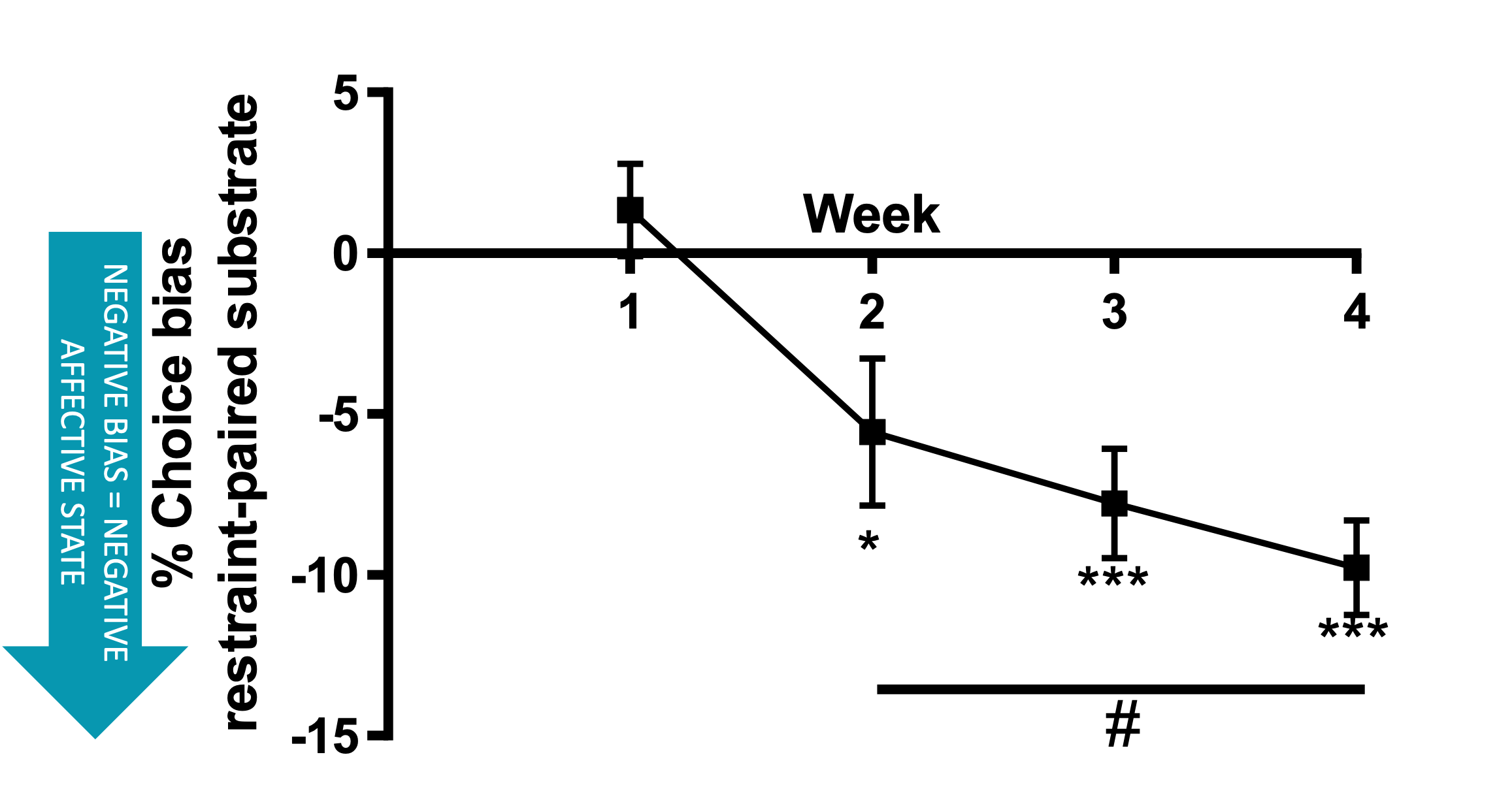
The negative affective state induced by restraint stress increases with each successive experience. These data were obtained using the affective bias test (see additional resources) with affective state quantified weekly following an acute restraint stress.
The response of animals to repeated restraint varies with some continuing to show overt signs of distress (active coping) and others appearing calm but exhibiting learned helplessness(passive coping).These data suggest there are negative welfare implications relevant to the whole population irrespective of coping strategy.
Mouse Handling
Mice find tail handling aversive. Alternative methods using cupping or tube handling avoid using the tail. Animals can also be moved from the tube to the hand or vet bed to facilitate health checks or regulated procedures.
Mouse handling method - tube
This video shows a mouse being tube handled and then returned to the cage.
Mouse handling method - cup
This video shows a C57 black mouse being cup handled from the cage and transferred to a pieces of vet bed then cup handled back to the cage.
Mouse handling method - tube to hand transfer
This video shows a mouse being tube handled from the cage and then onto the hand.
Rat Handling
Rats should not be handled by the tail, their heavy bodies mean this method can lead to injury. They are docile and readily become habituated to human handling. Young rats are very unlikely to bite unless fearful or experiencing pain. Older animals can be more unpredictable if not accustomed to handling. For most situations animals can be handled around their shoulders. Tube handling can also be used and may be needed for animals which are not habituated. This method is also useful for handling pregnant dams or those with pups.
Rat handling method - lifting from shoulders
This video shows a male wistar rat being picked up from the cage around the shoulders then moved to the arm.
Rat handling method - tube
This video shows a group of female rats being picked up from the cage using a tube method.
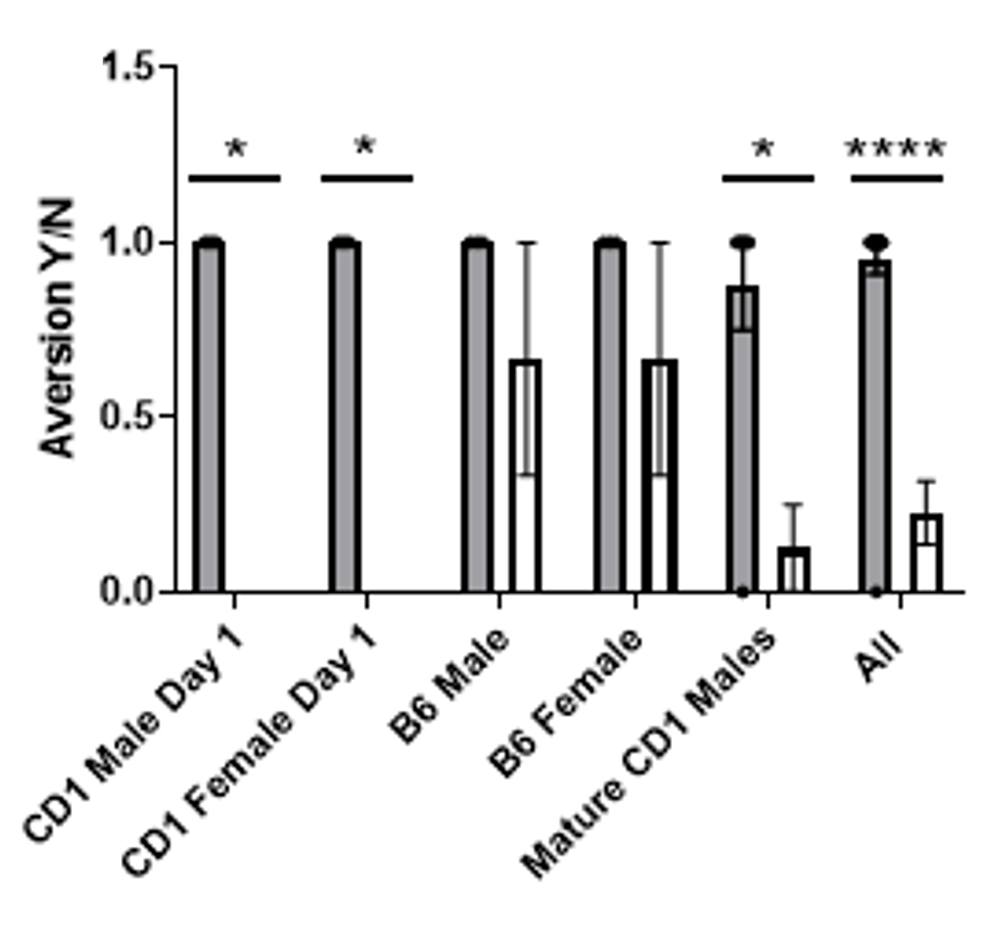
Refined handling methods in mice reduce aversion on release.
Mice handled using the tail (solid bar) exhibit an escape response when released from the hand suggesting aversion, but this is reduced when animals are handled using our refined method (open bar).
Methods which can be used to facilitate restraint in mice but which avoid the use of tail handling
Conventional restraint methods used for mice involve holding the animal by the base of the tail. As tail handling is known to be aversive to mice, we habituate mice to cup handling and directly restrain from this position on the arm or after placing the mouse onto vet bed.
The video examples show different combinations of handling and restraint methods which can be used for mice to enable substance administration via subcutaneous and intraperitoneal routes of administration. These same methods can be used for other procedures where the animal needs to be restrained.
Substance Administration Mice - Arm scruff
This video shows a mouse being restrained using a scruffing method but avoiding the use of tail handling. In this example, the mouse is cup handled onto the arm and then restrained.
Substance Administration Mice - SC standard
This video shows a mouse being restrained for subcutaneous dosing. The animals is cup handled onto the arm, restrained using a scruff method and then transferred to the table to permit SC dosing.
Substance Administration Mice - SC vet bed
This video shows a mouse being restrained for subcutaneous dosing. The mouse is cup handled onto vet bed then restrained using a scruffing method.
Substance Administration Mice - SC vet bed with tube
This video shows a mouse being tube handled from the cage to hand before being placed onto vet bed and restrained for intraperitoneal dosing.
Methods which can be used in rats to facilitate substance administration via the intraperitoneal or subcutaneous route of administration, but which avoid physical restraint
These modified handling methods avoid using firm and restrictive physical restraint. These are best achieved by habituating the animals to handling then introducing them to the dosing position with the time the animals is held for gradually increased. This training can be integrated with the initial handling habituation and can be introduced as soon as animals are accepting basic handling. Using a syringe without a needle can be useful as part of acclimatisation.
Substance Administration Rats - IP with needle - Example 1
This video illustrates a modified restraint method which can be used for intraperitoneal injections in rats. The animals is lightly restrained in a natural position.
Substance Administration Rats - IP with needle - Example 2
This video shows a female wistar rat being restrained for intraperitoneal injection training. No needle is used but the animal is held in the correct position and an empty syringe used to practise the handling method.
These videos illustrate some potential issues with our modified handling method and intraperitoneal dosing in rats and how the manage them.
With very large animals, it can be more difficult to keep the dosing hand clear of the animal’s mouth and care should be taken to avoid having the dosing hand where it could be bitten should the animal experience pain from the injection.
Substance Administration Rats - IP with needle - Example 3
This video shows a large rat being dosed using a mock intraperitoneal injection where the handlers hand is close to the animal’s mouth and could be bitten.
Some rats can learn to avoid the handling position for dosing and require a bit more patience and small adjustments to the position being used. It is nearly always possible to achieve a safe handling position without using a firm physical restraint.
Substance Administration Rats - IP with needle - Example 4
This video shows rat trying to avoid being held in the dosing position and the handler making adjustments to how it is held to facilitate the injection.
Some rats learn to reverse to avoid being held in the position for intraperitoneal dosing, but this can be overcome by making a small adjustment to the handling method and using the little finger of one hand to hold the rat’s hind leg.
Substance Administration Rats - stop from reversing
This video illustrates how to hold a rat that is trying to avoid the handling method by reversing. Using the little finger of the restraining hand to hold the hind leg, the animal is not able to reverse.
A common mistake with this handling method is tipping the animal too far back. If the animal is tipped backwards it will react to this unnatural position by struggling making it difficult to inject. To ensure clear access to the injection site, gentle pressure is used to ‘compress’ the animal into a curved but upright position and this makes it easier to see the injection site but also maintains the animal in a more natural position.
Substance Administration Rats - stop from reversing
This video shows why it is important not to tip the rat backwards into an unnatural position from which it will try to escape and struggle.

Position for intraperitoneal dosing in rats.
The rat is held in a ‘compressed’ position leading to a relaxed belly which reduces discomfort during injection.
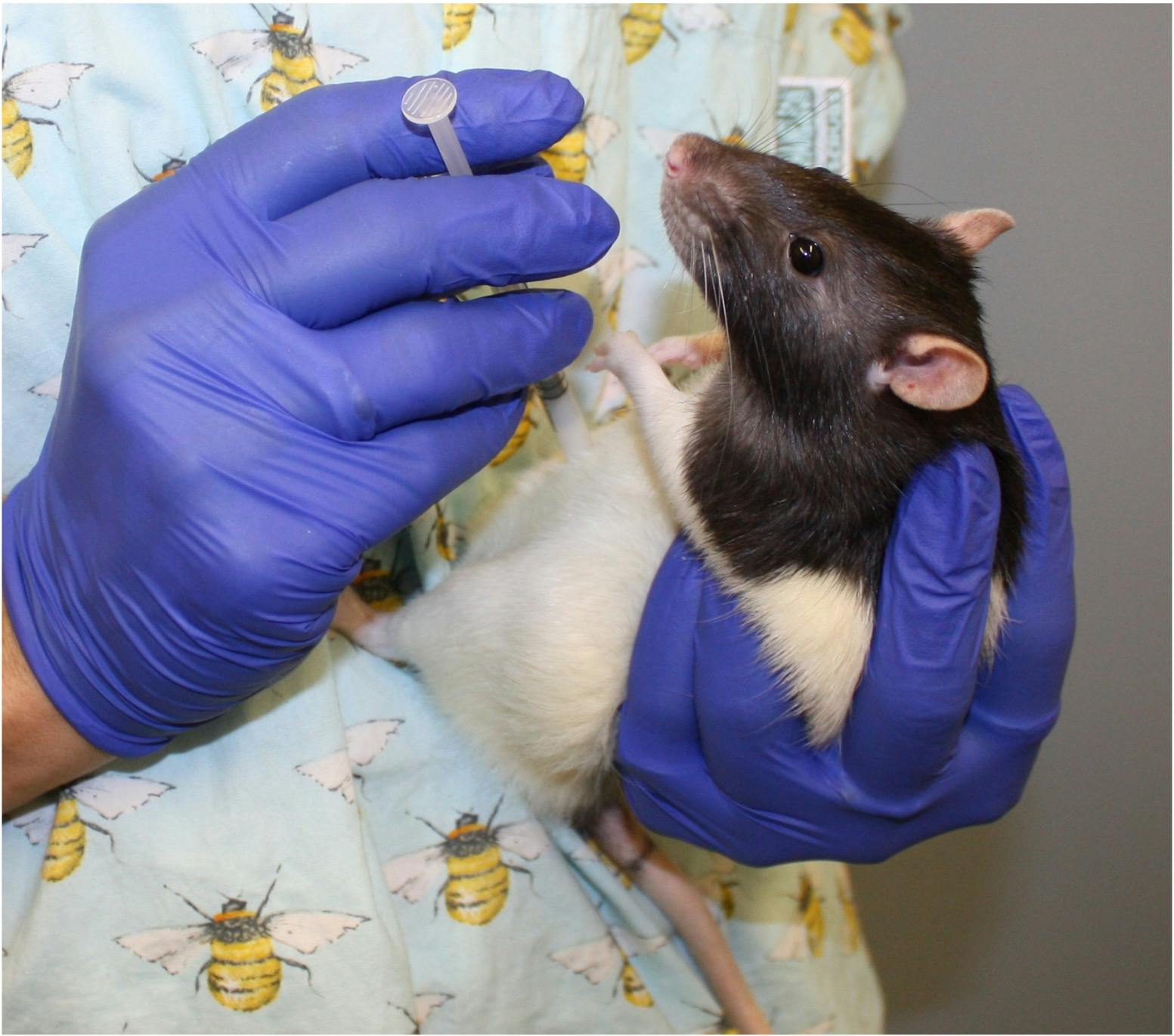
This image illustrates the injecting hand being too close to the animal’s head and a risk of being bitten.
During training and habituating the rats to the dosing position, the technique is refined before progressing to injections.

This animal is held with a slightly firmer grip which incorporates holding the back leg which is useful if animals are reversing and can also provide more control of the animal’s position. The animals is still held in the ‘compressed’ position to reduce pain associated with the intraperitoneal injection.
Substance Administration Rats - SC vet bed
This clip demonstrates a method for subcutaneous dosing in rats with the animal held gently on vet bed.
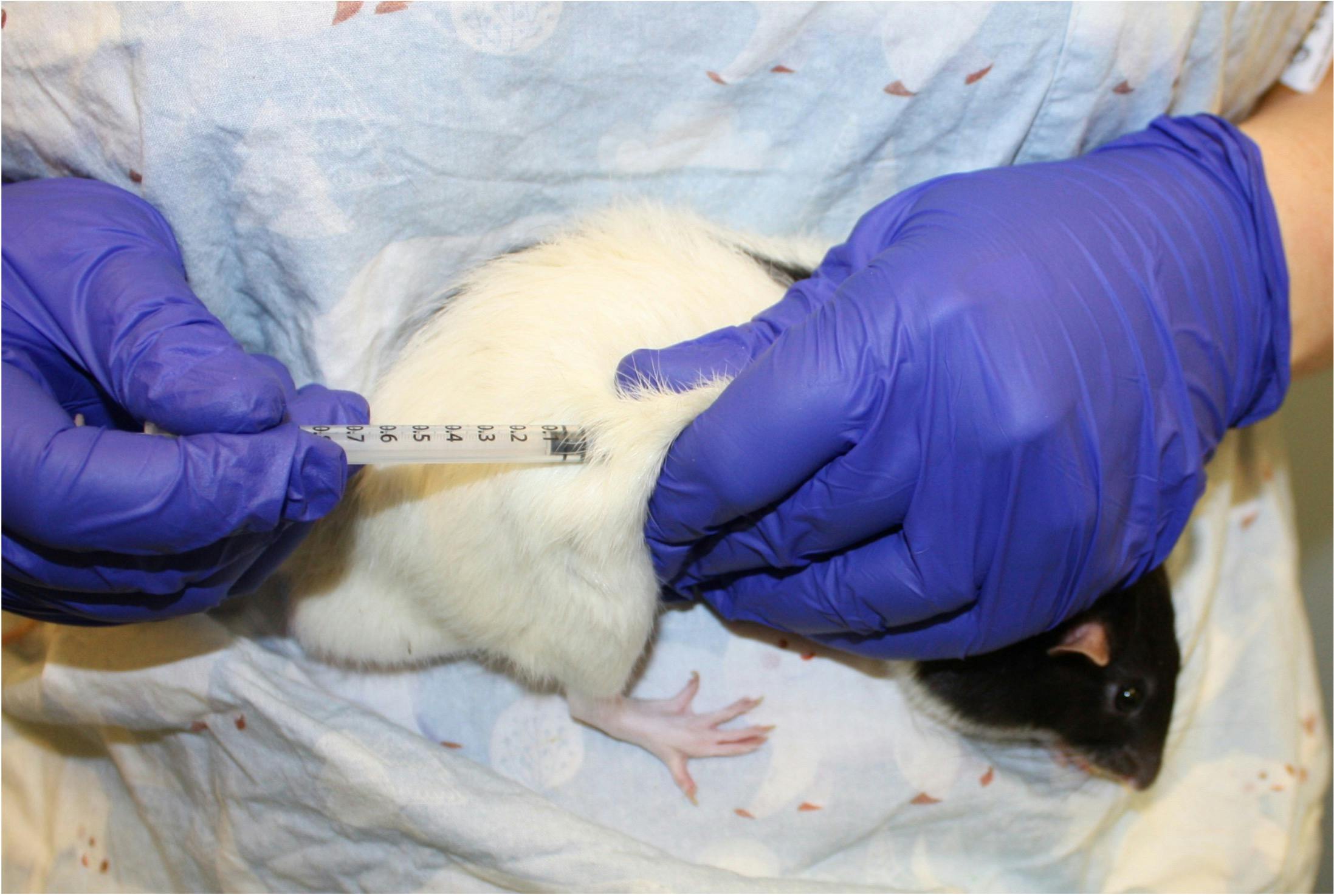
Subcutaneous dosing can also be carried out by gently holding the animal against the body.
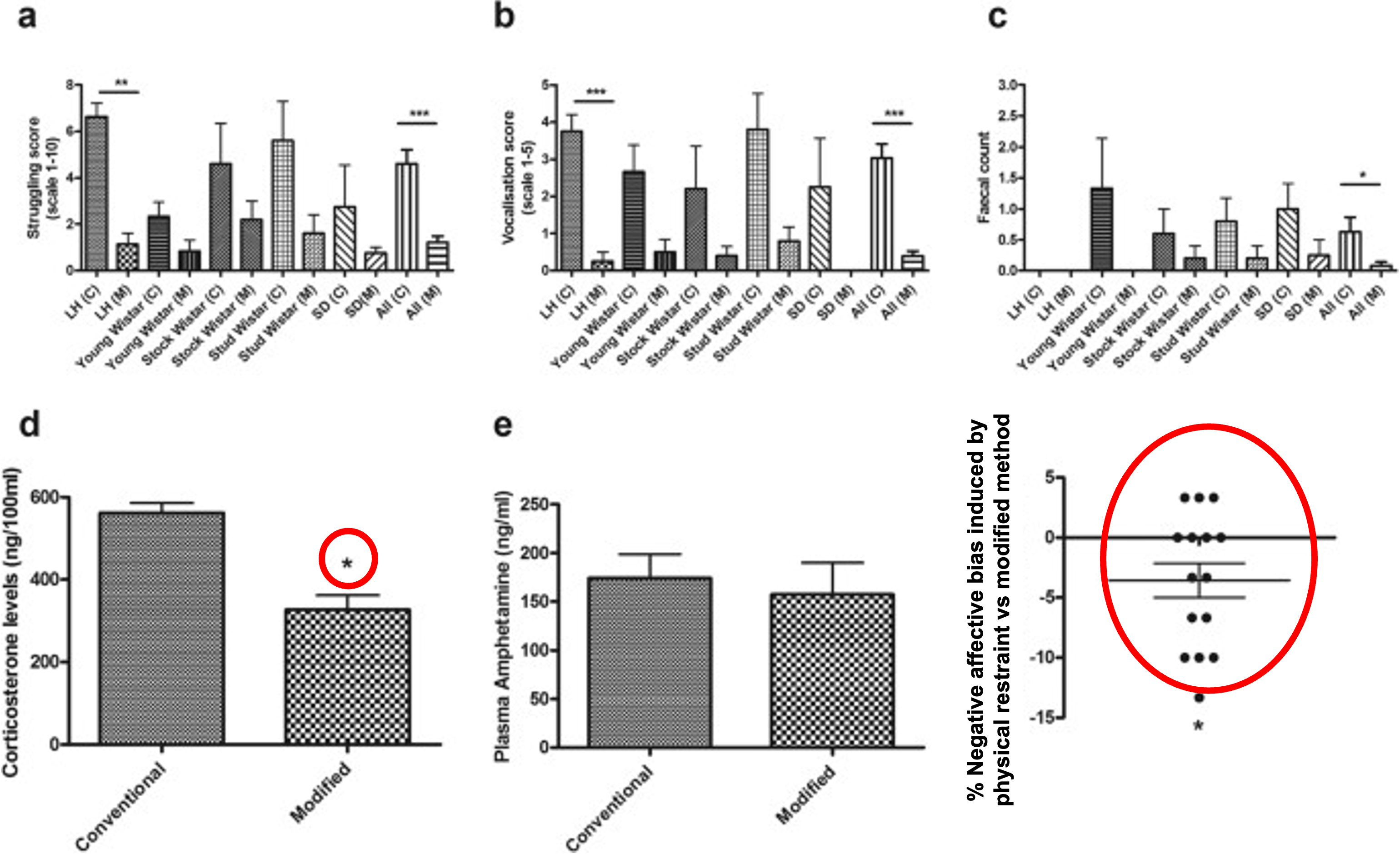
Rats handled using the modified handling method for intraperitoneal injection show less overt signs of distress (panels a-c), lower plasma corticosterone (panel d) but with no effect on the pharmacokinetics of the drug (panel e). Using the affective bias test we could also show that conventional restraint methods generate a more negative affective state (panel f) (Stuart et al., 2015, https://www.nature.com/articles/srep14288).
Dosing in palatable solutions
Substance Administration Mice - oral self-administration
This video illustrates dosing via a syringe using a palatable vehicle in mice in the home cage.
Substance Administration Rats - oral self-administration - Example 1
This video illustrates dosing via a syringe using a palatable vehicle in rats held in the hand.
Substance Administration Rats - oral self-administration - Example 2
This video illustrates dosing via a syringe using a palatable vehicle in rats in the home cage.

Mice prefer to receive the palatable solution in their cage. Presenting the syringe when they are protected by their shelter or tube can be helpful during training. Only very well-handled mice will drink whilst being held.
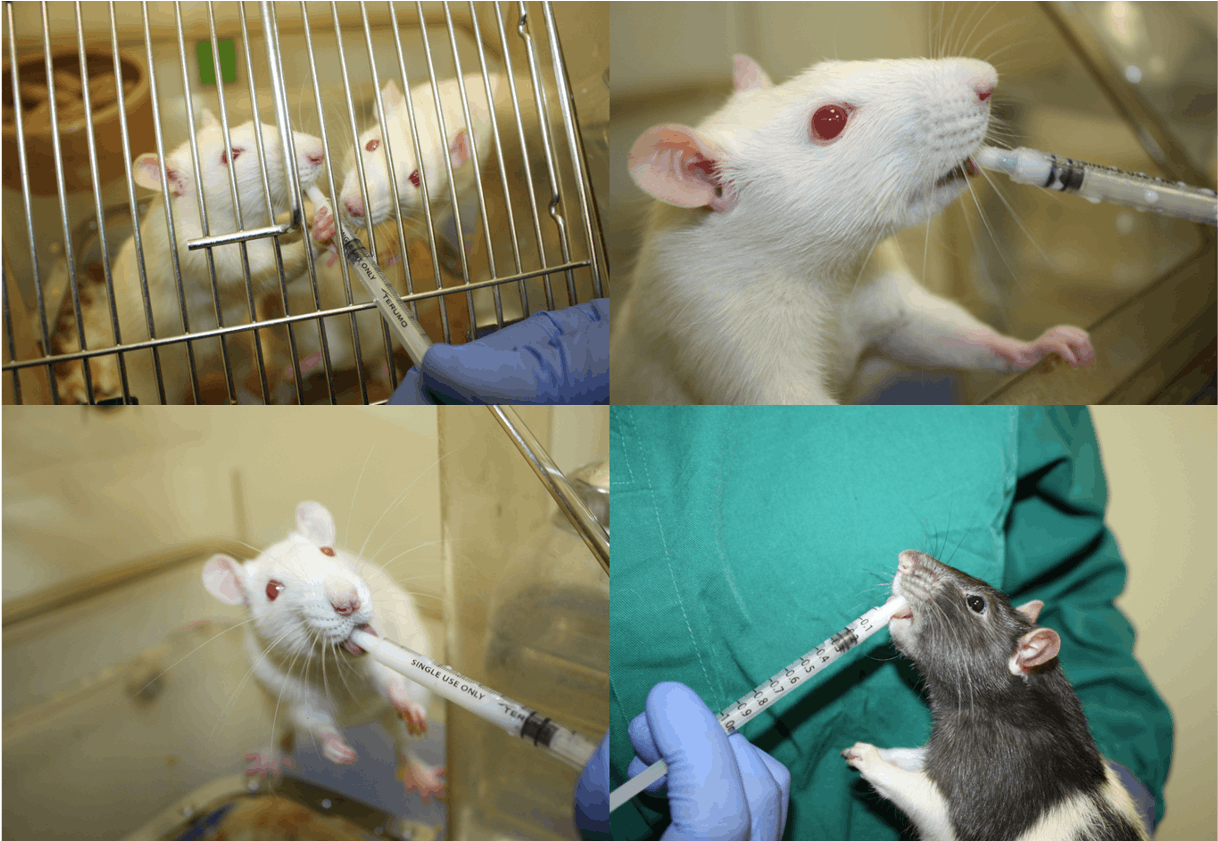
Rats can be habituated to palatable solutions in pairs or groups but will quickly start to compete to access the syringe with the dominant animal(s) limiting access for others in the cage. After initial habituation, rats are best trained and dosed individually either in a cage or held in the hand.
Training for oral dosing using palatable solutions
Stage 1: Acclimatisation to palatable vehicle in home cage
The palatable solution is first introduced to the animals in the home cage with their cage mate(s) if group housed. Rats may take from the syringe immediately but both mice and rats can be first introduced to the reward by placing a small amount into the cage in a dish or onto a suitable surface in the cage e.g. on top of the shelter. This can be combined with habituation to handling with the palatable solution for oral dosing used as positive reinforcement.
Stage 2: Introduce the palatable solution from a syringe
Once animals are consuming the palatable solution from within the cage, the syringe can be introduced. This is done in the home cage with the cage mate(s) if group housed. For larger groups of animals or with very dominant individuals, it can be useful to use two syringes. The syringe can be introduced through the bars of the cage, or the cage lid removed and offered to the animals. Mice may initially prefer to drink whilst partly protected by their shelter or tube. Rats can quickly become very keen to drink and may try to take hold of the syringe. When training a group of animals, make sure all have had an opportunity to drink from the syringe. If any animals are reluctant to drink or are being pushed away by others, remove the cage mates and re-offer the syringe.
Once all animals are drinking from the syringe in the home cage, progress to individual administration either within the home cage (by removing the other animals) or by transferring to another cage. Rats will usually happily drink from a syringe whilst being held and can be removed individually for dosing.
Stage 3: Daily administration of the palatable solution maintained throughout the study
It is useful to continue to give animals daily (Monday – Friday) administration of the palatable solution in addition to any treatments. Regular access can help reduce any impacts of treatments which may be bitter in taste or could generate conditioned aversion. We have an ongoing programme testing different formulations for oral administration to overcome bitter tasting compounds and to optimise protocols to reduce conditioned aversion e.g. if a drug treatment causes nausea.
Guidance documents detailing oral dosing methods and our most recent developments can be found in our new voluntary oral dosing section.