

Voluntary Oral Dosing
This page provides support for the uptake of voluntary oral dosing as a refined route of administration for drug studies.
Introduction
Voluntary oral administration using palatable vehicles involves training rodents to willingly consume drug formulations mixed with palatable vehicles.
Compared with traditional administration methods like oral gavage or injections, voluntary dosing offers significant welfare and scientific advantages. It reduces handling stress, eliminates the risk of dosing-related injury, and minimises restraint. These factors may improve the validity of study outcomes by reducing stress-related variability. It also supports longitudinal studies by enabling repeated administration without inducing fear or aversion. Another example of a successful voluntary oral dosing protocol can be found here.
This page provides a step by step protocol supported by data-driven evidence for rapid uptake by rodents. It provides advice for commonly encountered problems, suggestions for palatable vehicles, and considerations for uptake of the method when starting a new study.
Protocol
Day 1:
Give animals free access to the palatable substance (examples provided in table 1) that you will be using in your drug study in their home cage. Recommended volumes are ~0.2ml per mouse and ~0.5ml per rat. This can be provided on a plastic petri dish or similar surface. Allow them to investigate in their own time for a period of up to 1hr. By the next day, the animals will know that the substance of safe and tasty and are more likely to approach the syringe to drink.
Day 2:
Mice
If group housed, separate mice into dosing cages. Make sure the same dosing cage is used for the duration of the study. Ensure the dosing cages contain a place to hide such as a shelter or tube. If single housed, the mouse can remain in the home cage during dosing.
Place the syringe through the bars of the cage lid in a place that is accessible to the mouse. The syringe should contain palatable vehicle of the volume required for the drug study. Keep the syringe still and wait for the mouse to approach. Do not follow the mouse around with the syringe. The mouse may initially sample the vehicle and run away before returning. This is part of normal mouse behaviour and will reduce over time.
Rats
As above. However, rats may not need separate dosing cages, they can be dosed at the same time using two syringes. If rats become competitive for a particular syringe, one rat can be briefly removed while the other consumes the palatable vehicle.
Day 3 – 5:
Repeat day 2, rodents will consume the palatable vehicle more rapidly and will have a shorter latency to approach the syringe.
When commencing the drug study, dissolve your drug into your chosen palatable vehicle and administer at a volume proportional to the animal’s body weight.
Once the drug study has commenced, animals should be presented with another syringe of ~0.2ml for mice and ~0.5ml for rats at a second timepoint (usually late afternoon post-test session) containing the palatable vehicle only. This should reduce the chance of the animals developing a negative association with the palatable vehicle.
We show that this protocol works across a range of commonly used mouse strains (figure 1).
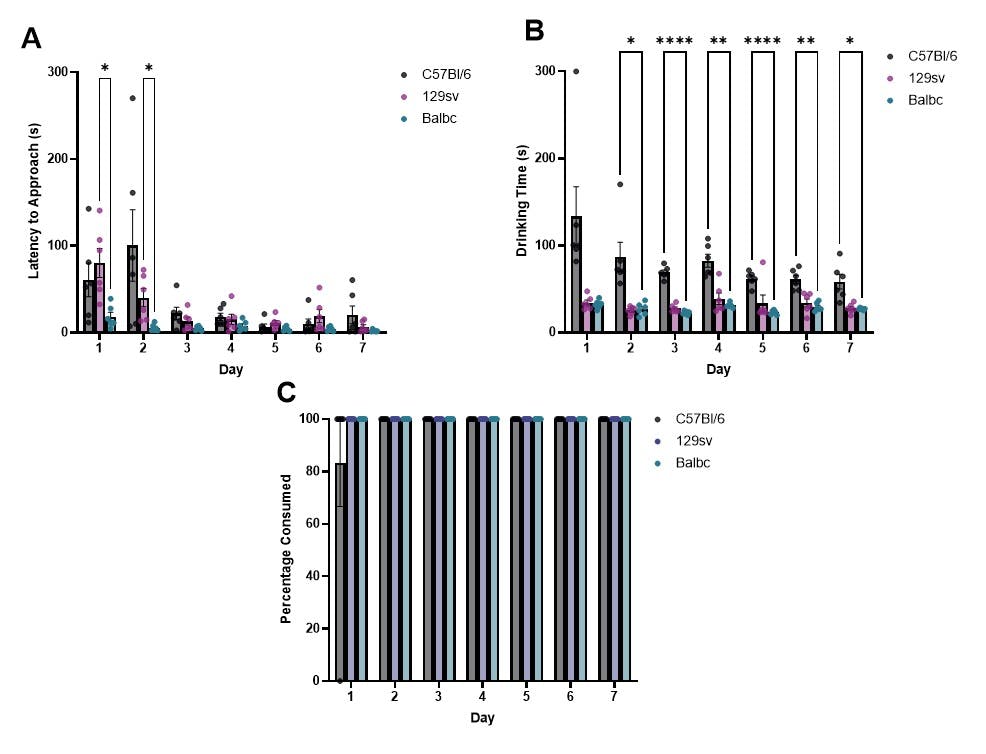
Figure 1. A While C57bl/6 mice initially show a longer latency to approach the syringe, this strain difference disappears within 2 sessions. C57bl/6 mice also show an improvement in drinking time over sessions. B After one session, time taken to consume the contents of the syringe fell to below 100 seconds across all strains. C57bl/6 mice showed the longest drinking times compared to other strains. C All mice reliably consume all liquid from the syringe within 1 session.
A habituated mouse drinking from a syringe
When mice are fully habituated to voluntary oral dosing they will readily approach the syringe and rapidly consume the vehicle. In some cases, pairing syringe presentation with a sound to alert the mouse to experimenter presence such as rustling the food can increase latency to approach syringe as shown here.
Rat dosed outside of the home cage
When fully habituated, rats do not necessarily need to be dosed inside a cage (home cage or other). This can be useful when one rat is more dominant than the other and tries to drink from multiple syringes when presented in the home cage. Note that individual differences will apply and you should judge whether this is the best approach for each rat.
Rat dosed within the home cage
An example of a rat being dosed within the home cage.
Suggested palatable vehicles
Below is a table (table 1) of tried and tested palatable vehicles to facilitate voluntary drug consumption. We have included both sugar and non-sugar-based vehicles to help align to individual study objectives. Please ensure that your chosen palatable vehicle is suitable for your study and does not interact with experimental variables.
Unless otherwise stated, where percentages are denoted, the remaining vehicle is standard tap water.
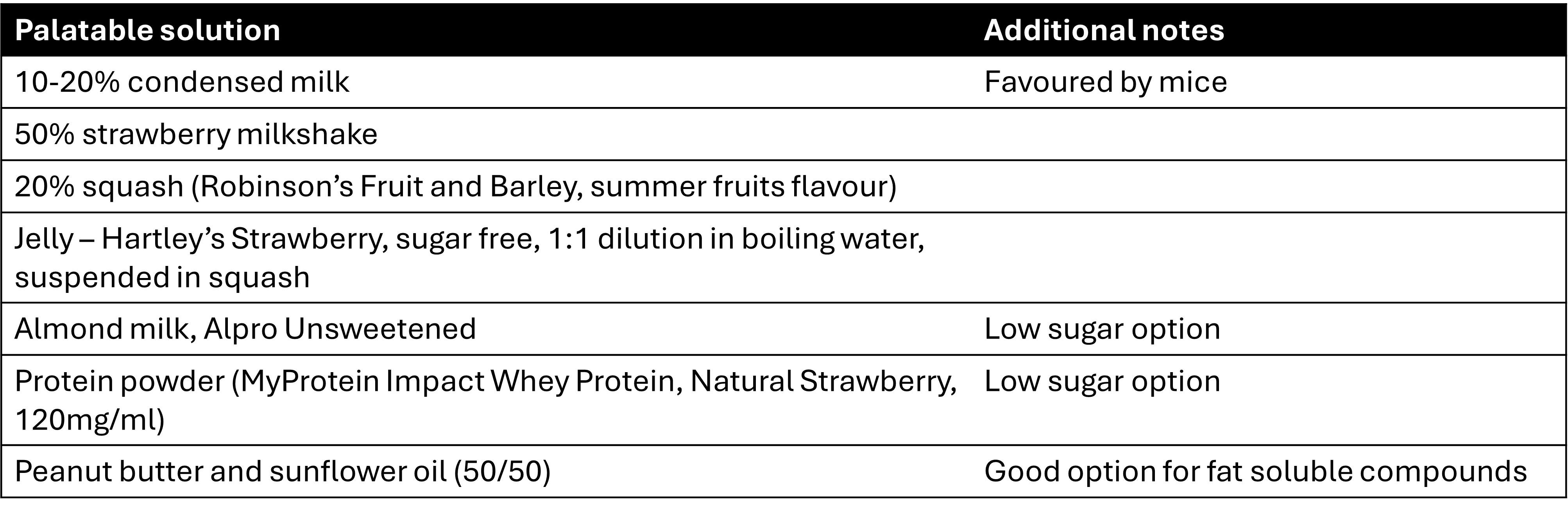
Table 1. Examples of palatable vehicles for voluntary oral dosing studies.
Tackling palatability and conditioned aversion
Some drugs may not be palatable enough to facilitate voluntary oral consumption in the standard palatable vehicles. In addition, conditioned aversion may occur if a drug induces a negative affective state in the animal, e.g., it causes nausea, discomfort, stress or alters emotional state. In this case, the animal may form a negative association with consuming the drug. This can be mitigated with using several different options.
Use of a masking agent which helps to neutralise the bitter taste of drugs
We show that the use of two different masking agents, Bitter Drug Powder (BDP) and Masking Mixture (MM) mask the bitter taste of Quinine which is known to have a strong and aversive taste in both rats and mice (figure 2). Quinine alone increases drinking time and decreases percentage of liquid from the syringe consumed compared to vehicle consistent with an aversive taste. This effect is rescued by the addition of BDP and MM. If a masking agent is used, the rodent should be habituated to it as part of the standard habituation protocol for palatable vehicles. Additionally we show that over time, rats show conditioned aversion to venlafaxine, evidence by reduced % consumed and increased drinking time over sessions. This change over time is mitigated by the addition of masking agents (figure 3).
Using additional ‘washout doses’
The rodent should be given a second dose containing palatable vehicle only later on in the day, post-test session. This can help to prevent a negative association forming with consumption from the syringe.
Use of different palatable vehicle flavours
Different flavours of a palatable vehicle e.g., chocolate, strawberry and banana milkshake can be used to prevent a negative association forming with any one flavour. It is important to habituate the rodent to all flavour options to avoid neophobia if using this method.
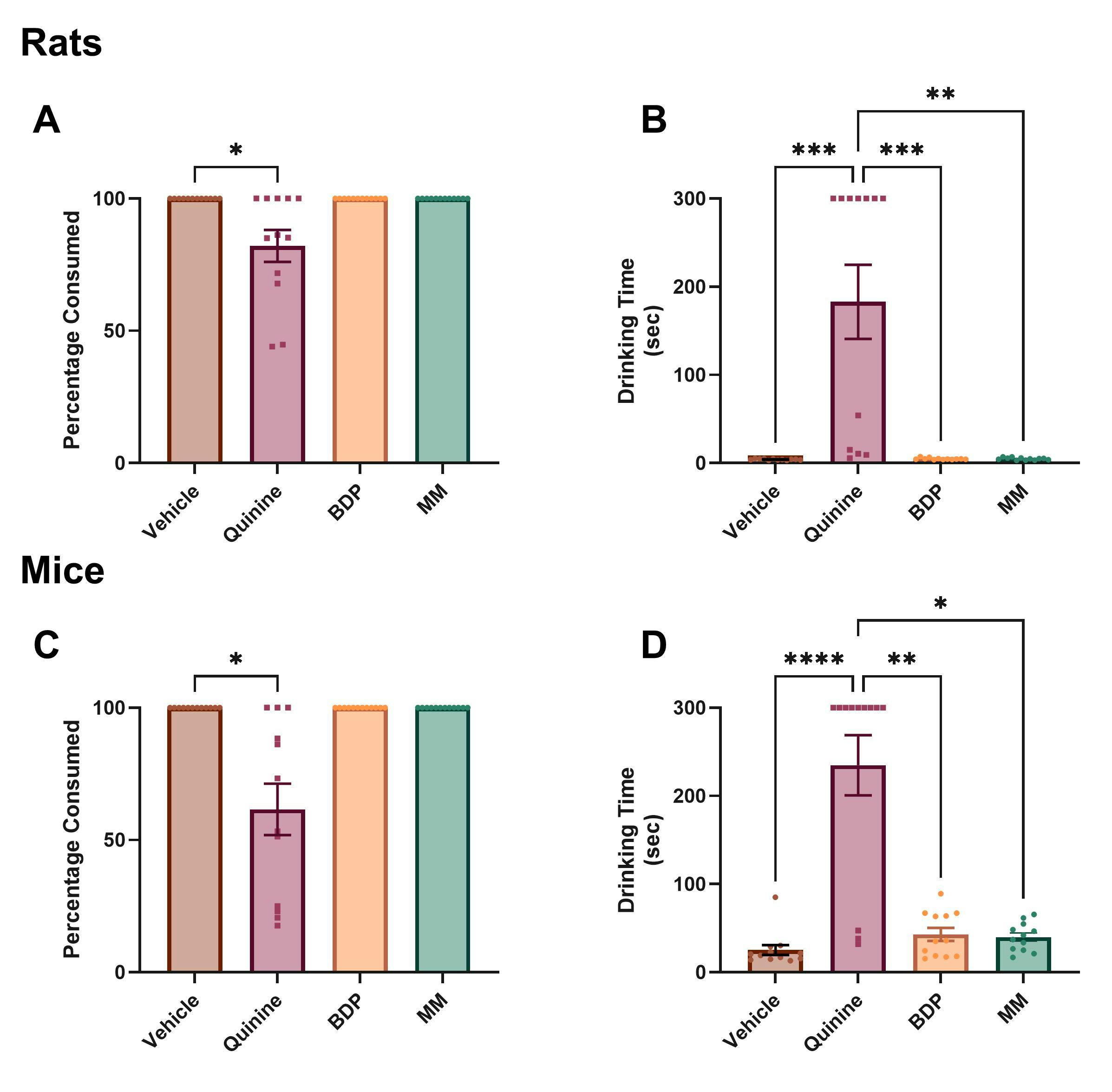
Figure 2. A Quinine is a bitter agent that reduced percentage of palatable vehicle consumed in rats. This effect was blocked by two different masking agents. B Quinine also increased time taken to consume the palatable vehicle from the syringe. This effect was blocked by these masking agents. C & D These effects were also found in mice. BDP= bitter drug powder, MM= masking mixture.
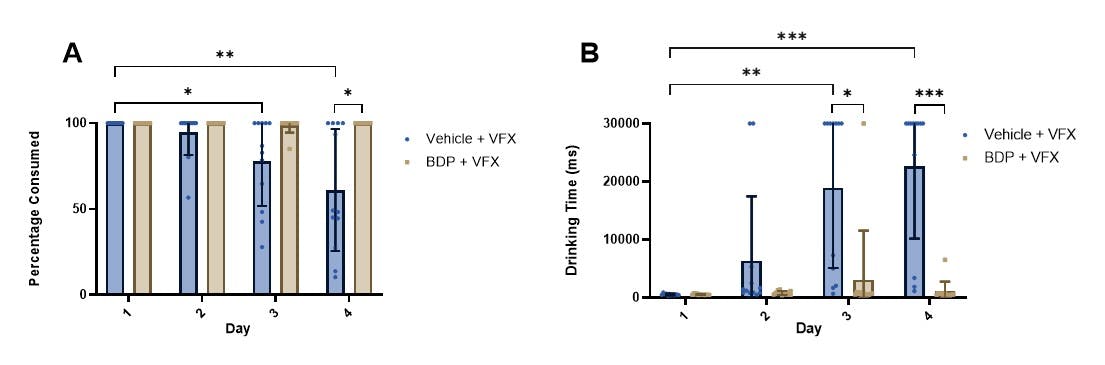
Figure 3. A The percentage consumption of VFX reduces over time, but this effect is blocked by BDP. B Drinking time for VFX increases over time, but this effect is blocked by BDP. VFX- venlafaxine, BDP- bitter drug powder.
Considerations for Switching to Oral Dosing
It is important to be aware that switching to voluntary oral dosing from other routes of administration such as intraperitoneal (i.p) or sub-cutaneous (s.c) methods may alter the pharmacology of the drug. For example, time to reach peak plasma concentration may be longer and therefore drug pre-treatment time may need to be altered depending on study question. It is also necessary to check whether your drug of interest has sufficient oral bioavailability. Doses may need to be altered to account for changes in drug pharmacokinetics. It is important to note that switching to voluntary oral administration does not necessarily change the desired effect of the drug (figure 4). A pilot study using an established readout may be designed conducted to confirm this in the context of your own experiment. Here, we show that switching to voluntary oral dosing does not impact on expected effect. Rats treated with the antidepressant venlafaxine via the intraperitoneal route induced a positive choice bias in the affective bias test (ABT). Rats treated orally with venlafaxine in a palatable vehicle showed a similar behavioural effect in the ABT. Here, pre-treatment time was not adjusted.
Not all drugs are suitable for oral administration. Some drugs may cause irritation of the oral-mucosal area or have poor palatability that cannot be masked by the suggested adjustments. In some cases, oral administration may not align with the study objectives. However, when starting a study, it is important to consider voluntary oral dosing as a potential refined alternative.
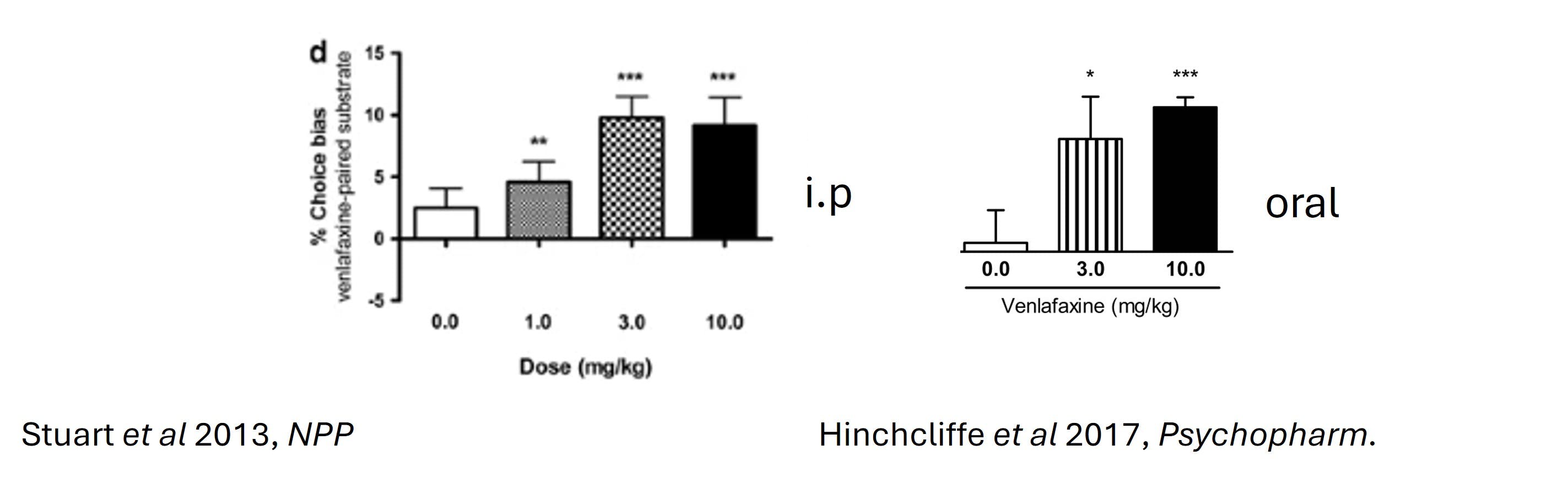
Figure 4. The behavioural effect of venlafaxine is consistent across dose routes.
Commonly Encountered Problems
Rodent will not approach the syringe
Ensure that you have completed the handling habituation protocol. If rodents are not habituated to experimenter interaction, they are less likely to approach the syringe which is typically placed towards the front of the cage. Ensure that the rodent has had the opportunity to freely consume the palatable vehicle within the home cage as per the first stage of the oral dosing protocol. This step is necessary to mitigate the impact of neophobia.
Mice become distracted from the syringe
It is normal for mice to disengage from the syringe and explore the home cage throughout the dosing period, this allows them to monitor their environment. Do not try to mitigate this by moving the syringe around to ‘follow’ the mouse through the cage as this can induce anxiety and lengthen dosing times. Leave the syringe in the same place and wait for the mouse to return. This behaviour reduces over time and with sufficient habituation.
Rodent shows limited interest in syringe
Ensure that you are using a vehicle that the rodent finds palatable (see table 1). If the rodent does not consume the vehicle while freely available in the home cage, switch to a different vehicle. Ensure there is some of the vehicle at the end of the syringe, the rodent will smell this and begin to lick. You may then start to depress the syringe. Ensure you depress the syringe at a rate consistent with licking rate. If the vehicle is dispensed too quickly, it can spray onto the rodent's faces which can create aversion to the syringe,
Rodent is allowing solution to drop on the floor
Ensure you are depressing the syringe to match the drinking rate of the rodent. Depressing the syringe too fast can lead to the rodent dropping the vehicle as it cannot keep up with the rate of release.
Mice show excessive facial grooming during dosing
As above, ensure you are depressing the syringe to match the rodent drinking rate. Splashing vehicle onto the rodent’s face/whiskers is aversive and can cause grooming which can lead to delays in dosing. Alternatively, the rodent may find the vehicle irritating. Make sure to check the pH of the vehicle and consider whether oral dosing is the most appropriate route if this persists.
You can use the flowchart in figure 5 to work out when to progress your animals through the steps of habituation and start the study.
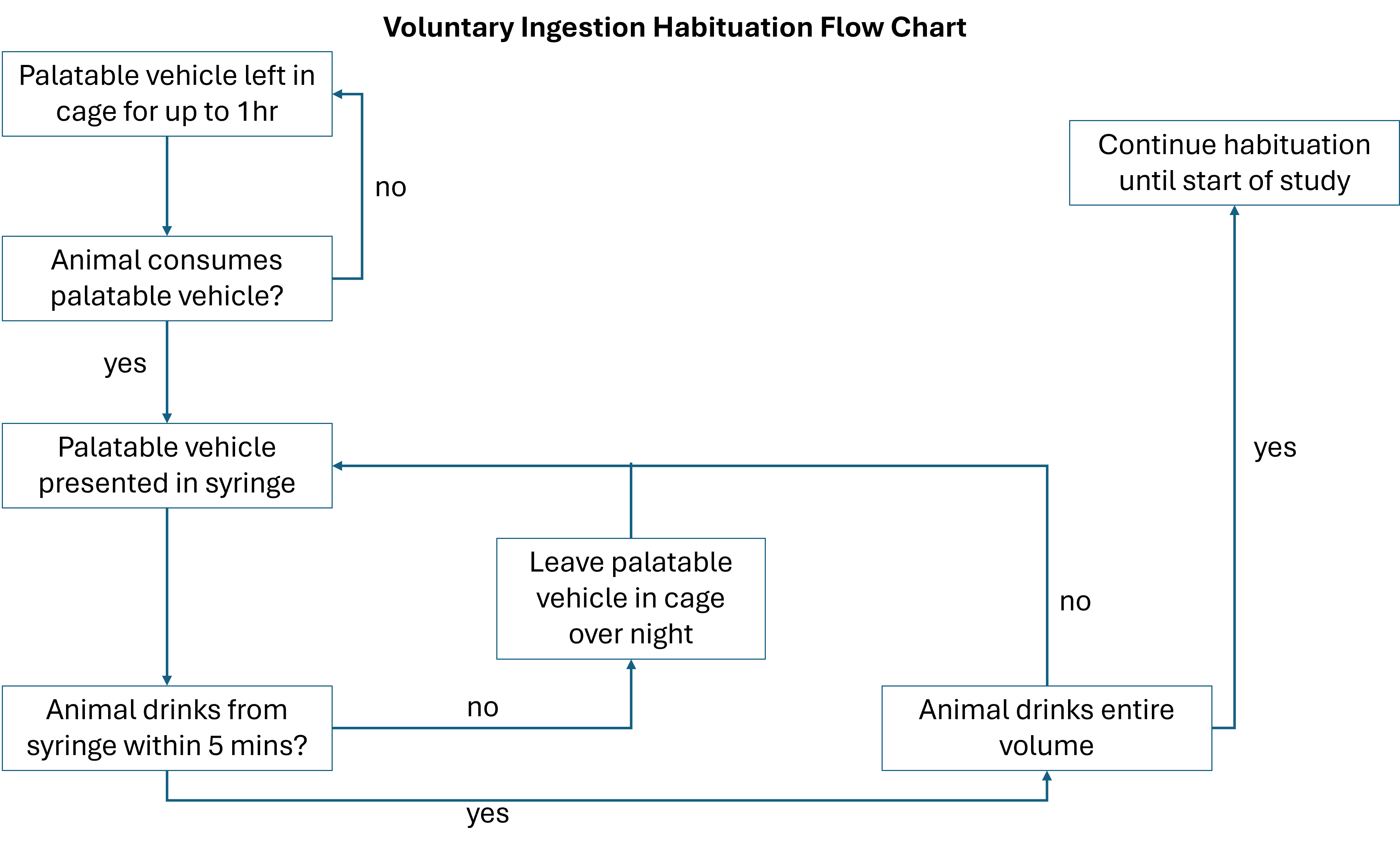
Voluntary Dosing Habituation Flow Chart